Electrostatic Force vs Intermolecular Force vs Covalent Bond VS Ionic Bonds : Online IGCSE Chemistry Class
ELECTROSTATIC FORCE VS INTERMOLECULAR FORCES VS COVALENT BONDS VS IONIC BONDS
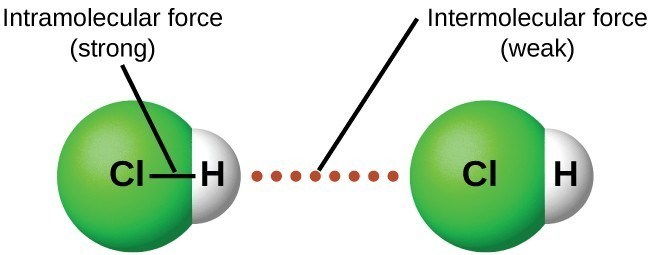
Intermolecular force binds the atoms making up a molecule or a compound. These are weak forces and only exist between molecules. Intramolecular force is a stronger force and it acts to hold atoms together to form ionic or covalent bonding.
There are two types of electrostatic forces in compounds or molecules, intramolecular forces that exist between the bonded atoms of a compound or a molecule, and intermolecular forces that exist between molecules